Home
Results of All
94 results of all were found
- Hospitals and Pharmacies | Fundamental sciences | Community Pharmacy | Selling and Marketing in Pharmacies | Essential Soft Skills | Management | Accounting | Legal affairs | Hospital pharmacy | Clinical pharmacy | Quality Control | Infection control | Storage | Mathematics
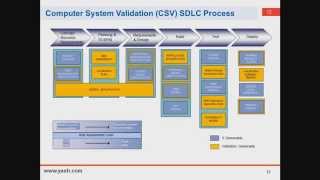
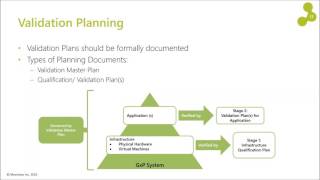
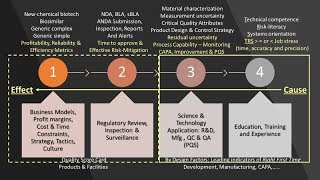
- Pharmaceutical Industry | Regulatory Agency Governance | Quality Systems | Laboratory Systems | Infrastructure: Facilities, Utilities, Equipment | Materials and Supply Chain Management | Sterile and Nonsterile Manufacturing Systems | Filling, Packaging, Labeling | Product Development and Technology Transfer | Safety and occupational health | Environmental safety | Pharmaceutical Validation
- Selling and Marketing | Selling skills | Marketing planning | Human Resources | Medical updates | Training skills | Regulatory affairs | Pharmacovigilance | Information Technology
- validation (43) | Cleaning Validation (10) | GMP (8) | Process Validation (8) | Computer System Validation (8) | Equipment Validation (7) | Arabic (7) | Equipment Management (6) | FDA (6) | WHO (5) | Analytical Method Validation (5) | Quality Assurance (3) | manufacturing (3) | formulation (3) | World Health Organization (2) | HVAC (2) | PIC/S (2) | Utilities (2) | synthetic (2) | sterile (2) | Community (2) | Pharmacy (1) | European Medicines Agency (1) | day (1) | Reference standards (1)
alot of knowledge are waiting for you
Powered by Mohamed Atia
Powered by Mohamed Atia